Written by Warren Whyte, PhD
The Food and Drug Administration (FDA) is increasingly emphasizing that oncology trial participants be as diverse as the U.S. population. In response, ConcertAI, through our ERACE program, has developed specific approaches that align attributes of our real-world data, AI SaaS technologies, and accelerated Digital Trial Solutions with this mission. This year’s SCOPE Summit provided the opportunity to present these approaches throughout the conference and in a keynote presentation. These emerging data solutions and digital tools are a critical part of bringing transformational medicines to patients who need them most, and we believe life sciences organizations that embrace the solutions that enable diversity in clinical trials will be leaders in advancing health equity more broadly.
The approaches we’ve evolved take the following steps:
- Use of real-world data at population scale, inclusive of clinical, medical claims, and social determinants of health (SDoH) to set diversity targets for specific programs and better understand peoples’ lifestyles, needs, and potential challenges to participating clinical trials;
- Use of digital technologies to develop and optimize study protocols; classify sites with diverse populations aligned to specific studies; and identify investigators for targeted engagement/enrollment; and
- Engage patient advocacy groups, community-based organizations (CBOs), and Community Outreach and Engagement (COE) leaders that can provide complementary clinical research education, health literacy, and awareness to communities.
The first step evaluates demographics, SDoH, and clinical information of racial/ethnic groups within various diseases using a combination of data sources to create “disparity heatmaps.” These customized reports reflect easy-to-digest analyses of areas and regions of interest to get deeper understandings of cancer disparities, including:
- An epidemiological breakdown of diseases prevalent in various zip codes, with benchmarking at local, state, and country level.
- Diseases creating a disproportionate burden, at what rate, and to which specific subgroups or subpopulations.
- Near real-time heatmaps, in specific areas where potential research sites are located, that support site-specific engagement strategies and advanced identification of potentially eligible patients.
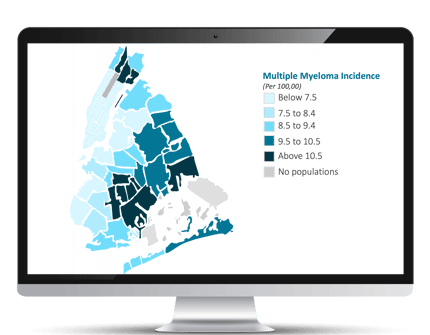
The second step evaluates specific real-world data solutions, integrating clinical, medical claims, and SDoH data using advanced analytics to identify potential clinical trial sites approximate to regions with identified disparities and with populations potentially eligible for a specific study design. Examples of the criteria include: (a) larger populations of racial, ethnic, and economic groups, including African American, Asian, American Indian, Native Hawaiian, and Hispanic, and (2) treating oncologists with current or prior research experience.
Given recent work demonstrating that many clinical trial criteria negatively affect enrollment of racial/ethnic groups, ConcertAI’s approaches use RWD and artificial intelligence (AI) to intelligently relax or tighten eligibility criteria to improve the enrollment size and diversity of the enrolling population. Diversity enrollment targets should be established based on the disease risk within racial/ethnic groups rather than the U.S. Census population, particularly when the distribution of the racial/ethnic group within a disease is higher than Census.
The third step evaluates community-based organizations located in proximity to regions of identified disparities. The primary activities of these organizations focus on health care, including cancer prevention, diagnosis, and treatment improvement. An effective engagement campaign requires working with these organizations, alongside patient advocacy groups and COE Leaders at NCI-designated Comprehensive Cancer Centers, to develop projects, community engagement activities, and community events designed to promote and increase participation in clinical trials; assist in the development of recruitment plans for specific trials; and assist in the development of protocols for specific trials. There can be social, psychological, and historical resistance when it comes to minority involvement in medicine. Thus, much of this community work would be dedicated to addressing the concerns that have caused this resistance.
Insights gleaned from these approaches can inform digital-based strategies to reduce health inequities among populations with existing disparities and create greater access to clinical trials for underrepresented patients. ConcertAI now runs a workshop, at no cost to biopharma sponsors on these approaches and tools. Please contact us here for more information or to schedule a date.

