Regulatory Research
External Control Arms for Timely Drug Approvals
ConcertAI’s deep, custom abstraction of patient real-world data and decades of scientific expertise helps clients through the regulatory process of designing and submitting successful External Control Arms.

Regulatory-Grade

Linked Claims, Genomics Datasets

Full-Service Consultation

Custom Abstraction of Specific Variables
Reasons for
External Control Arms
Request a Demo
Rare Cancers
Identify patients with rare solid tumors or hematologic malignancies
Competitive Trial Space
Limit susceptibility to competitive trial recruitment by leveraging retrospective samples
Patient Criteria
Oversample critical subgroups of interest for deeper analyses
Trial Completion
Find, recruit, and analyze control patients in months rather than years
INFOGRAPHIC: EXTERNAL CONTROL ARMS CAN BRING LIFE-SAVING CANCER DRUGS TO MARKET FASTER
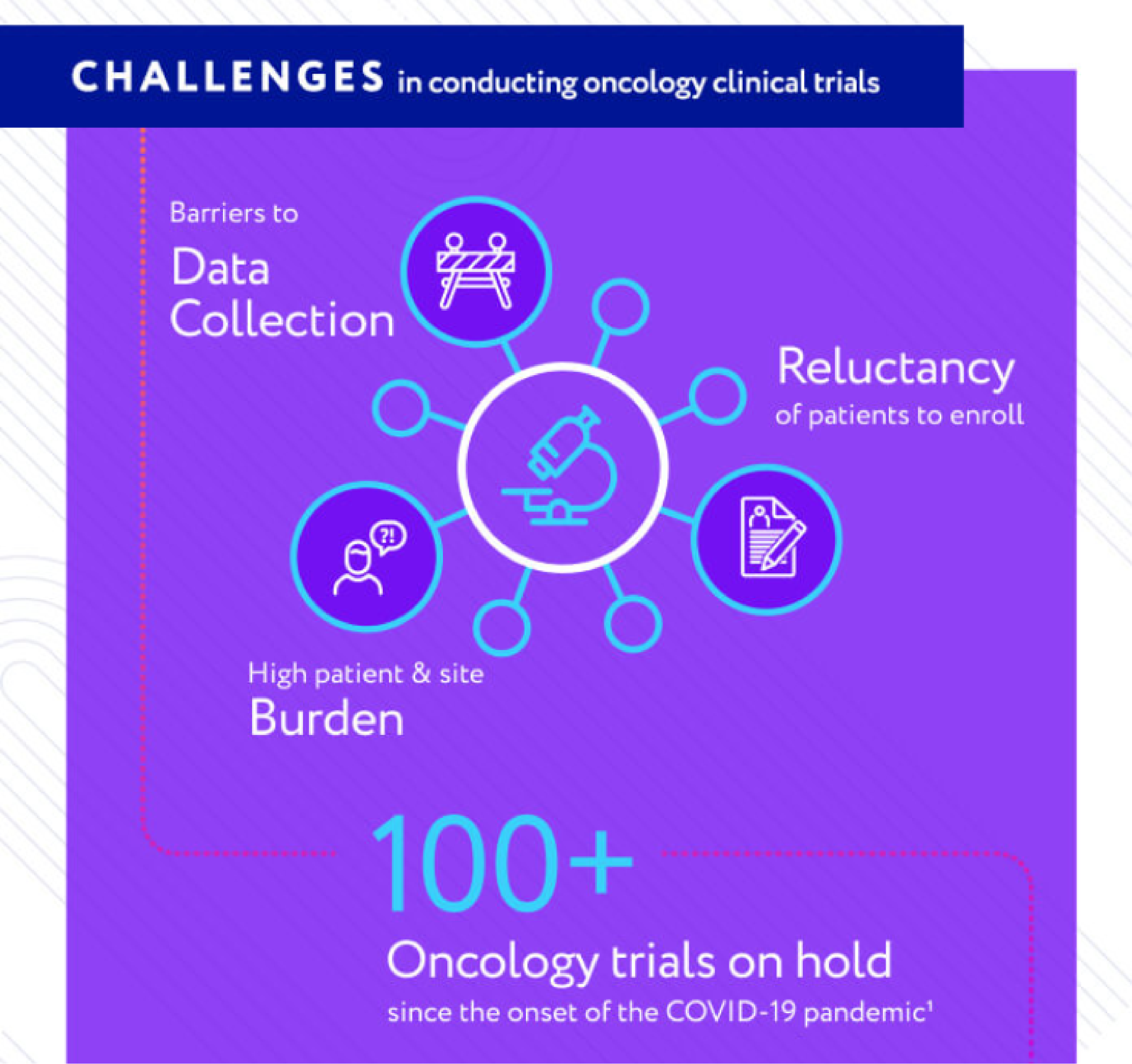
Clinical Trial Challenges
Clinical trials are expensive and challenging in ordinary times. COVID-19 has made this situation much more difficult, negatively impacting clinical trial activities and delaying life-saving treatments. In response, the FDA has acted recently to create an environment to encourages novel trial designs where external control arms can thrive.
EXTERNAL CONTROL ARMS
In-Depth, Regulatory-Grade RWD and Scientific Expertise
- Pre-Approval
- Post-Approval
- Inform clinical trial design and feasibility with our interactive cohort matching tool
- Conduct propensity score matching or frequency matching with demographic and clinical characteristics matched to clinical trial patients
- Rescue underperforming trials when the RCT is having difficultly accruing patients in a standard of care cohort
- Satisfy market requirements for fast-tracked, breakthrough applications or newly approved treatments coming to market with limited comparative data
- Fulfill post-authorization safety assessments, surveillance, and/or efficacy evaluations required by regulators
- Conduct studies for label expansion and new indications
- Find new dosing schedules and routes of administration
- Dynamically track the ECA cohort from diagnosis through discharge, end of record, or death
- Generate persuasive comparative evidence to demonstrate product value
INFOGRAPHIC: EXTERNAL CONTROL ARMS CAN BRING LIFE-SAVING CANCER DRUGS TO MARKET FASTER
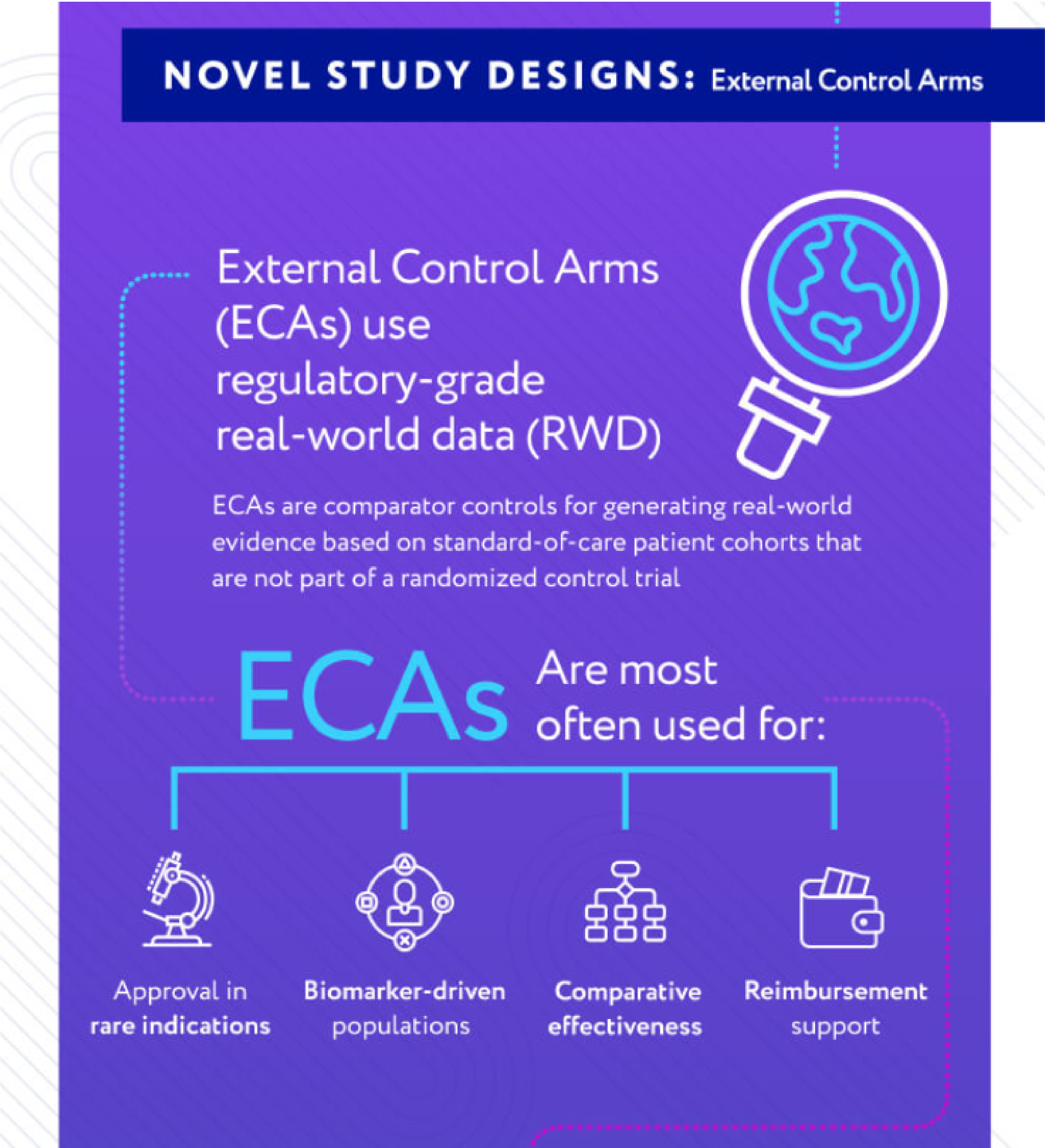
Novel Study Designs
External control arms use real-world data (RWD) to enhance data from single-arm trials. They can speed up patient accrual and reduce costs. Results include:
- 50% reduction in patient accrual needs
- Ability meet fast-tracked designation timelines
- Increased trial efficiency
- Reduced site and patient burden
- Decreased risk
EXTERNAL CONTROL ARMS
Working with a one-stop partner for deep, reliable RWD and expert research and analytics services can lead to:

Timely Treatment Assessments

Faster Drug Approvals

Reduced Site and Patient Burden
Customer Value for External Control Arms
Request DemoClinical Development Leaders
Rapidly generate insights into standard of care and areas of unmet need with access to thousands of on-demand and custom abstracted data elements
HEOR, Market Access and Medical Affairs Executives
Leverage RWD for post-marketing analyses, comparative effectiveness analyses, and reimbursement submission needs for new indications/label expansions
Epidemiologists
Understand patient and disease characteristics
Translational Medicine Leaders
Evaluate the performance of existing therapies and identify risk factors associated with poor outcomes