INDEPENDENT, SCALABLE AND SPECIALIZED
Real-World Data for Rapid Insights
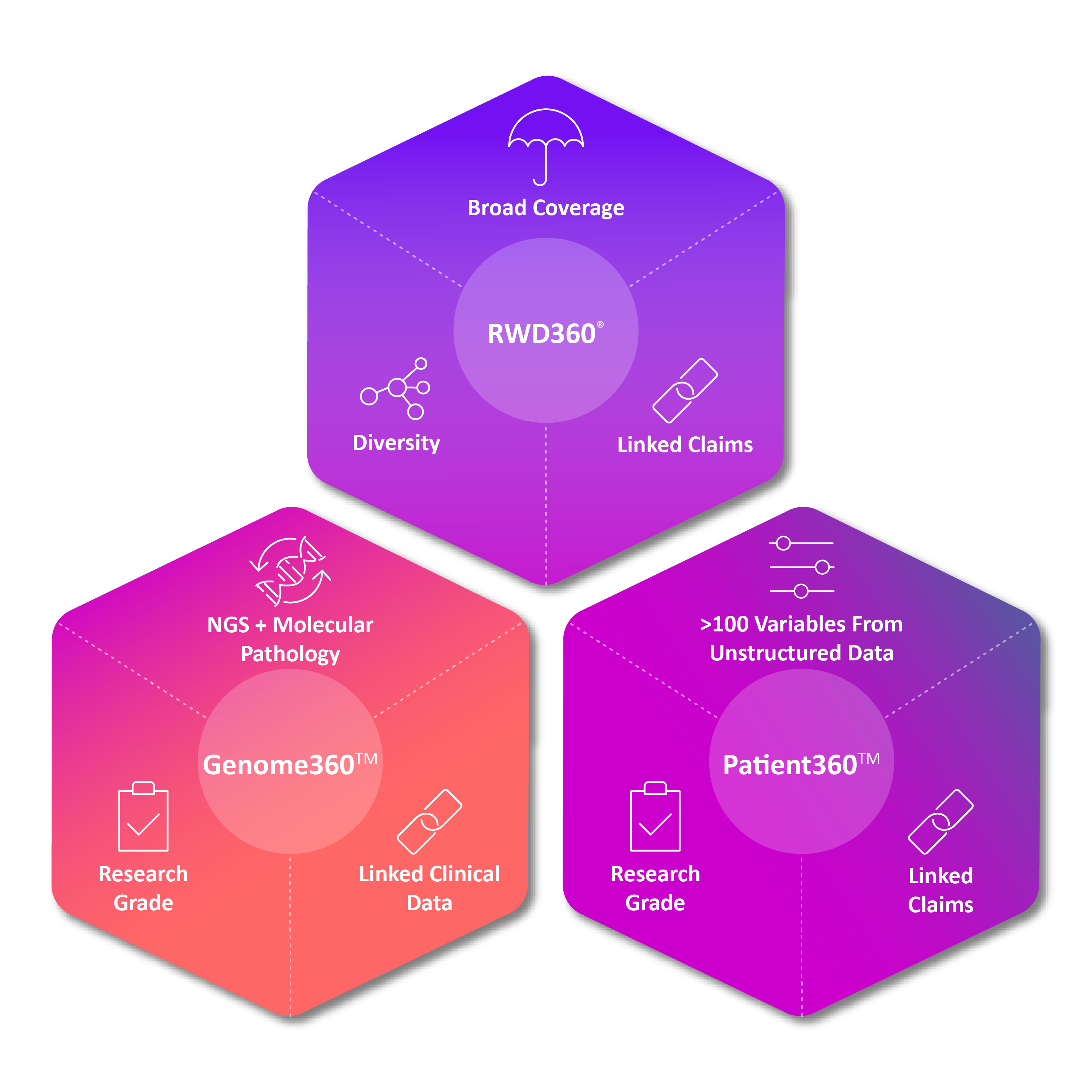
ConcertAI’s rich suite of independent real-world data products are fit-for-purpose to meet a variety of research needs and use cases across the enterprise
EMR Systems
Clinical EMR-Open Claims Overlap
Structured Variables
Abstracted Variables
Average Longitudinal Follow-up Time
Biomarkers
AI Models
What Data Trained Your AI?
LEADING INNOVATORS
On-Demand Data for
Accelerated Research
- Post-Approval
- Clinical Development
- Comparative effectiveness in real-world populations compared to clinical trials
- Study feasibility analyses
- Patient population comparisons and analytics
- Characteristics of patient journey and treatment sequencing over time
- Adverse event detection and evaluation
- Treatment patterns and analysis
- Biomarker data impact on treatment selection and outcomes
- External control arms
- Clinical trial protocol development and optimization
- Identification of undiagnosed and under-treated patient groups
- Study feasibility analyses
- Patient population comparisons and analytics
- Characteristics of patient journey and treatment sequencing over time
- Adverse event detection and evaluation
- Treatment patterns and analysis
- Biomarker data impact on treatment selection and outcomes
Data Products
AI-Enriched Real-World Data Products

All Solid Tumors and
Hematologic Malignancies

Broad Geographic
Coverage

AI-Enriched and
Sophisticated Models
Reasons for RWD360™
Request InformationCompare Characteristics
Compare demographic characteristics based on different treatments.
See Treatment Sequencing
See how treatments and drug class exposures being sequenced in a cohort.
Combine Clinical Criteria
Sub-cohort insights with a combination of clinical criteria like ECOG, early-stage disease, histology, blood-test results, BMI, etc.
Customer Value for RWD360™
Clinical Development Teams
Rapidly generate insights into standard of care with on-demand access to thousands of data elements and accelerate innovation and drug discovery timelines.
Predictive AI and Data Scientists
Explore AI-driven models from predicting outcomes to classifying critical patient sub-cohorts, ConcertAI is at the forefront of innovation demonstrated by our wide range of papers in this rapidly evolving area.
Business Development Leaders
Centralize enterprise data needs with the ability to easily combine disparate datasets and shorten forecasting workflows with richer clinical data at the population level.

Deepest Industry Abstraction

On-Demand Datasets
Reasons for Patient360™
Request InformationIdentify Study Feasibility
Identify base rates of clinical characteristics, disease severity measures and treatment outcomes in real world treatment to inform the feasibility of using RWD as a comparator arm.
Post-Approval RWD
Conduct effectiveness outcomes studies in specific populations of interest and compare to clinical trials.
Track Safety
See adverse events experienced by real-world populations and compare to clinical trials.
Customer Value for Patient360™
Clinical Development Leaders
Rapidly generate relevant insights into standard of care, develop novel research studies, and shorten drug development timelines.
Post-Approval/Epi-Safety Executives
Bring automation and data science to post-marketing analytics, comparative effectiveness studies, and risk surveillance.
Brand Managers
Assess and monitor treatment journeys and adherence patterns for strategic development and post-launch planning
Which Cancers Can I Research?
Research Study
Performance of Proxy Measures Compared with Direct Observation of Progression-Free Survival
See results from findings presented at ISPOR 2020 and ASCO 2020 on the performance of proxy measures against directly-observed measures of PFS by using structured and unstructured information from ConcertAI’s Oncology Real-World Database.
See Research Study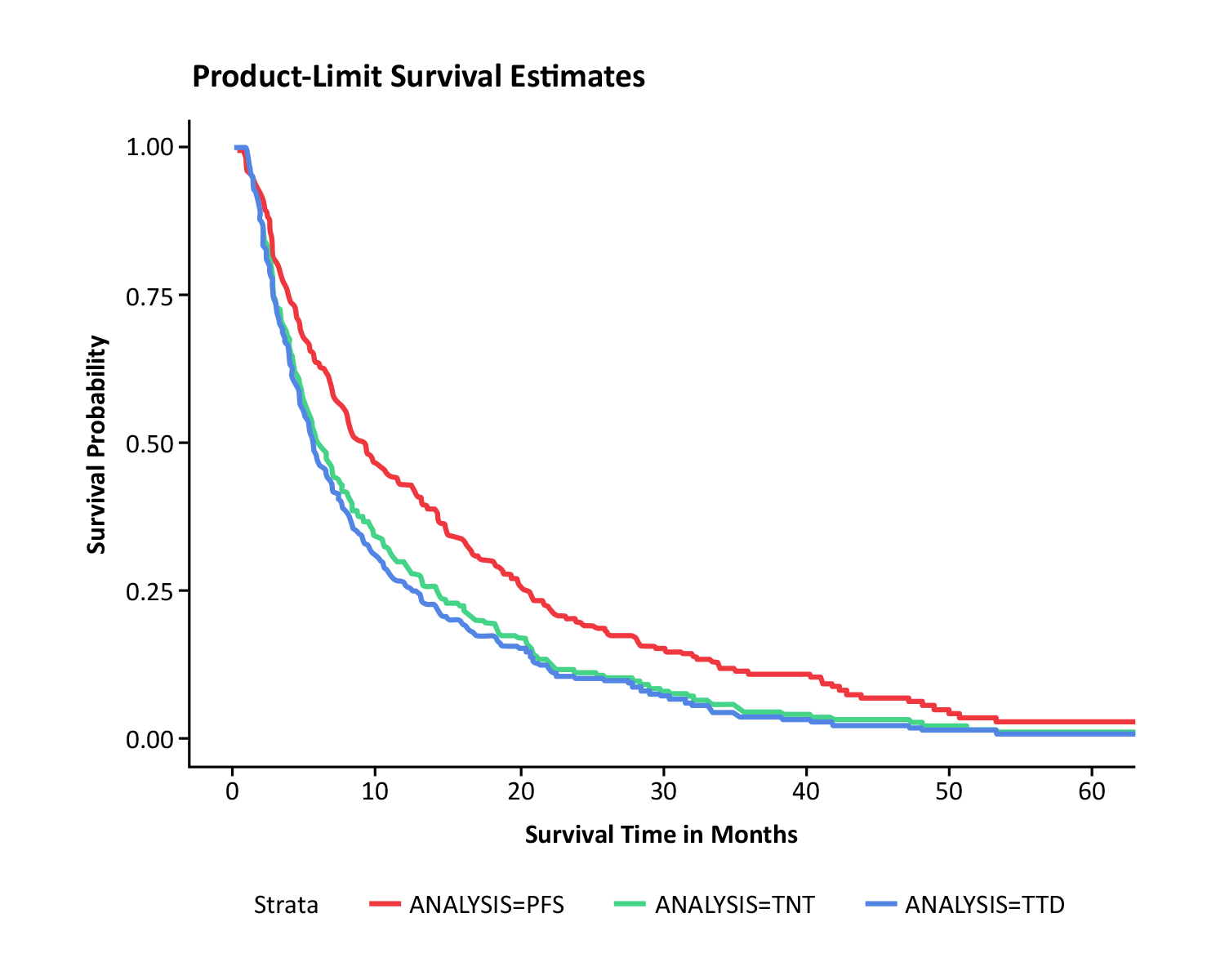

Full NGS Panel

Linked Clinical-Genomic

Deepest Industry Abstraction
Reasons for Genome360™
Request InformationBiomarker Testing Results
See biomarker testing, results, and timing and understand trends and how they change over time.
Identify Patients by Gene Variants
Identify patients by any type of variant in a given gene, e.g. mutation, amplification, rearrangement, etc.
Assess Treatments and Outcomes
Assess how testing (and additionally, positivity) affects treatment selection and outcomes.
Customer Value for Genome360™
Clinical Development Leaders
Rapidly generate relevant insights into standard of care, develop novel research studies, and shorten drug development timelines.
Post-Approval/Epi-Safety Executives
Bring automation and data science to post-marketing analytics, comparative effectiveness studies, and risk surveillance.
Brand Managers
Assess and monitor treatment journeys and adherence patterns for strategic development and post-launch planning.

Oncology and HEOR Expertise

Extensive Knowledge of RWD

Biostatistical Design and Execution

Regulatory Support and Publications
See Publications
Our scientific team regularly publishes with our clients based on research using custom data across a variety of therapeutic areas.
Reasons for ConcertAI Custom Data
Request InformationPrecise Patient Journeys
Create bespoke datasets to explore the real-world treatment landscape
Depth of Information
Customize datasets for granular insights into specific outcomes and endpoints
Longitudinal Datasets
Commission datasets to function as EMR registries


